
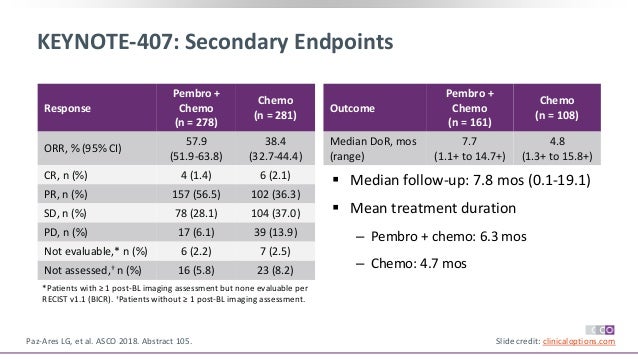
Keynote 407 trial#
Proffered Paper: Biomarker Analysis And Updated Results From The Phase 3 PROpel Trial Of Abiraterone (Abi) And Olaparib (Ola) Vs Abi And Placebo (Pbo) As First-Line (1L) Therapy For Patients (Pts) With Metastatic Castration-Resistant Prostate Cancer (mCRPC). Study EV-103 Cohort K: Antitumor Activity Of Enfortumab Vedotin (EV) Monotherapy Or In Combination With Pembrolizumab (P) In Previously Untreated Cisplatin-Ineligible Patients (Pts) With Locally Advanced Or Metastatic Urothelial Cancer (la/mUC). Hammel.īelzutifan, A Hypoxia-Inducible Factor-2α Inhibitor, For Von Hippel-Lindau (VHL) Disease-Associated Neoplasms: 36 Months Of Follow-Up Of The Phase 2 LITESPARK-004 Study. Wu.Įxtended Overall Survival Results From The POLO Study Of Active Maintenance Olaparib In Patients With Metastatic Pancreatic Cancer And A Germline BRCA Mutation.

Keynote 407 plus#
Tucatinib Plus Trastuzumab In Patients (Pts) With HER2-Positive Metastatic Colorectal Cancer (mCRC): Patient-Reported Outcomes (PROs) From Ph 2 Study MOUNTAINEER. Dent.Īdditional Analyses Of MOUNTAINEER: A Phase 2 Study Of Tucatinib And Trastuzumab For HER2-Positive mCRC. HRQoL With Neoadjuvant Pembrolizumab + Chemotherapy Vs Placebo + Chemotherapy, Followed By Adjuvant Pembrolizumab Vs Placebo For Early-Stage TNBC: Results From KEYNOTE-522. Seven-year OS data from the Phase 3 SOLO-1 trial evaluating maintenance LYNPARZA in patients with advanced BRCA-mutated ovarian cancer, following first-line platinum-based chemotherapy (Presentation #517O) and final OS results from the Phase 3 PAOLA-1 trial evaluating maintenance LYNPARZA in combination with bevacizumab in patients with advanced ovarian cancer following first-line platinum-based chemotherapy and bevacizumab (Presentation #LBA29) įirst-time data from Cohort K of the Phase 1b/2 EV-103/KEYNOTE-869 trial evaluating PADCEV ® (enfortumab vedotin) in combination with KEYTRUDA as first-line treatment for patients with cisplatin-ineligible unresectable locally advanced or metastatic urothelial cancer (Presentation #LBA73), which reported positive topline results for the primary endpoint of objective response rate earlier this year įirst-time data from NCI-sponsored SWOG S1801, a Phase 2 study of neoadjuvant versus adjuvant KEYTRUDA for clinically detectable and resectable stage III to IV melanoma, to be featured in Presidential Symposium II (Presentation #LBA6). Key Merck and collaborative data at ESMO 2022:įive-year overall survival (OS) data from the pivotal Phase 3 KEYNOTE-189 and KEYNOTE-407 trials evaluating KEYTRUDA in combination with chemotherapy as first-line treatment for patients with metastatic non-small cell lung cancer (NSCLC) (Presentations #973MO and #974MO, respectively) Presentations will feature new or updated findings from Merck’s growing pipeline and broad portfolio of cancer medicines: KEYTRUDA WELIREG™ (belzutifan) LYNPARZA (in collaboration with AstraZeneca) LENVIMA ® (lenvatinib, in collaboration with Eisai) and ODM‑208, an investigational steroid synthesis inhibitor (in collaboration with Orion). “Notably, we look forward to sharing five-year survival results from the pivotal KEYNOTE-189 study that has established the foundational role of KEYTRUDA in the first-line treatment setting for patients with metastatic nonsquamous non-small cell lung cancer, and results from EV-103/KEYNOTE-869, a study of KEYTRUDA in combination with the antibody-drug conjugate, enfortumab vedotin, in patients with locally advanced or metastatic urothelial cancer.”
Eliav Barr, senior vice president and head of global clinical development, chief medical officer, Merck Research Laboratories. “We are proud to present longer-term survival data in patients with lung, ovarian, melanoma and head and neck cancers, as well as findings that reinforce the impact of our medicines in earlier stages of certain cancers and in new combinations,” said Dr. The breadth of data showcases the continued impact of Merck’s portfolio of oncology medicines and the potential of Merck's innovative oncology pipeline. Merck (NYSE: MRK), known as MSD outside of the United States and Canada, today announced that research spanning 16 different cancer types will be presented at the European Society for Medical Oncology (ESMO) Congress 2022 in Paris, France from Sept. Seven-year survival from SOLO-1 and final overall survival (OS) results from PAOLA-1 highlight role of LYNPARZA ® (olaparib) in first-line maintenance of advanced ovarian cancerįirst presentation of KEYTRUDA in combination with an antibody-drug conjugate (enfortumab vedotin) (Phase 1b/2 EV-103/KEYNOTE-869 Cohort K) Longer-term survival results underscoring role of KEYTRUDA ® (pembrolizumab) in multiple cancer types, including advanced nonsquamous non-small cell lung cancer (KEYNOTE-189)


 0 kommentar(er)
0 kommentar(er)
